

Aspartame
Aspartame is a low-calorie sweetener, approximately 200 times sweeter than sucrose (table sugar). It is made from L-Phenylalanine and L-Aspartic Acid — the building blocks of proteins — just like those found naturally in daily foods, such as milk, rice, meat, eggs and fish. It is digested by the body exactly the same way as these other proteins, which means aspartame brings nature material to the body.
- Download Product Brochure
| Form | Powder |
| Brand | G sweet |
| Packaging Size | 25 kg |
| Packaging Type | Box |
| Usage/Application | Beverages |
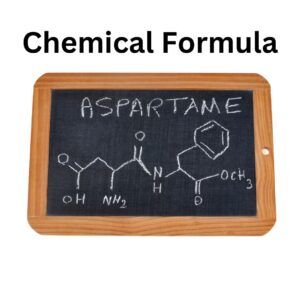
| Chemical formula | C14H18N2O5 |
| Molecular Formula | C14H18N2O5 |
| HS Code | 29242990 |
| CAS No | 22839-47-0 |
| Type | Sweetener |
Aspartame is a low-calorie artificial sweetener used in many sugar-free and “diet” food and beverage products. It is a compound made up of phenylalanine, aspartic acid, and methanol. Here are some key points about aspartame:
1. Sweetness:
– Aspartame is approximately 200 times sweeter than sucrose (table sugar). Due to its high sweetness intensity, only a small amount is needed to achieve the desired level of sweetness.
2. Applications:
– It is commonly used as a sugar substitute in a variety of products, including soft drinks, sugar-free or “diet” beverages, chewing gum, desserts, yogurt, and other sugar-free or reduced-calorie food items.
3. Caloric Content:
– Aspartame is low in calories because the body metabolizes it into components that do not contribute significantly to caloric intake. It provides sweetness without the caloric content of sugar.
4. Stability:
– Aspartame is sensitive to heat and breaks down into its constituent amino acids at high temperatures. Therefore, it is often not suitable for use in baked goods or products that undergo prolonged heating.
5. Phenylalanine Content:
– Aspartame contains phenylalanine, an essential amino acid. However, individuals with phenylketonuria (PKU), a rare genetic disorder, need to monitor their phenylalanine intake, as they lack the enzyme necessary to metabolize it properly.
6. Sweetening Power:
– Because aspartame is much sweeter than sucrose, it can be used in smaller amounts to achieve the same level of sweetness. This contributes to the reduction of calories in food and beverage products.
7. FDA Approval:
– Aspartame has been approved for use by numerous regulatory agencies, including the U.S. Food and Drug Administration (FDA), the European Food Safety Authority (EFSA), and others, when consumed within established acceptable daily intake (ADI) levels.
8. Use in Cooking:
– Aspartame is not suitable for cooking or baking at high temperatures, as heat can degrade its sweetness. It is best used in products that do not require extensive heat processing.
9. Dietary Considerations:
– Individuals with phenylketonuria (PKU) should be mindful of their phenylalanine intake and may need to limit their consumption of aspartame-containing products.
Aspartame is a widely used sugar substitute that has been extensively studied for its safety. However, individuals with specific health conditions, such as phenylketonuria, should be cautious about their intake of aspartame. It is important to follow regulatory guidelines and recommendations regarding acceptable daily intake levels.
The chemical formula of aspartame, a low-calorie artificial sweetener, is C₁₄H₁₈N₂O₫. It is a compound composed of carbon (C), hydrogen (H), nitrogen (N), and oxygen (O) atoms. Aspartame is a dipeptide, meaning it consists of two amino acids: aspartic acid and phenylalanine. The chemical structure of aspartame is:
H
/
H — N — C — C — O — CH₂ — C — H
\
O — NH — CH — C — H
\
O — H
In this structure:
– H represents hydrogen.
– C represents carbon.
– N represents nitrogen.
– O represents oxygen.
The aspartic acid and phenylalanine components give aspartame its sweet taste, as it is approximately 200 times sweeter than sucrose (table sugar). It is commonly used as a sugar substitute in various food and beverage products.
